
Testing Compliance in the 2023 Pharmacy Cleanroom
With the revisions to the 2019 USP General Chapter <797> Pharmaceutical Compounding – Sterile Preparations to potentially come into effect at some point in 2023, there is no better time than to sit down and look at your efforts to meet and maintain compliance in your pharmacy cleanroom.
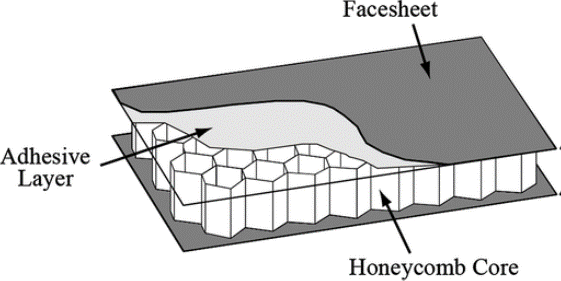
Modular Systems Vs. Stick Built:
As healthcare facilities begin to plan out a new pharmacy cleanroom, there are to two major options that are commonly considered. These options include Modular Systems and Stick Built. Within this article, we analyze these two approaches and weigh the true costs over the long-term.

Insanitary Conditions at Compounding Facilities and Prevention Methods
In November 2020, The FDA issued an Industry Guidance document geared towards providing information in helping pharmacy compounding facilities (as well as state regulatory agencies) understand some examples of what FDA considers to be insanitary conditions. We take a further look at a few of those examples and discuss on some prevention methods to keep and maintain a sanitary cleanroom environment.
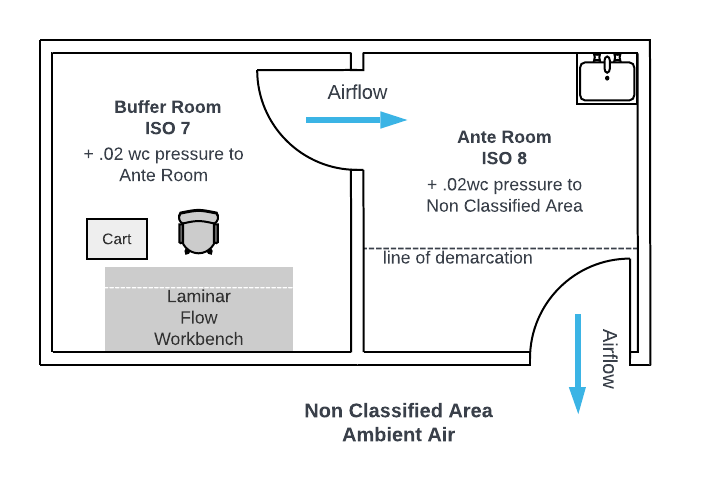
Pharmacy Cleanroom Differential Pressurization: Cascading Airflow Design
When creating separate rooms of different class, differential pressurization through a cascading effect enhances the performance keeping contamination from reaching the critical process environment. But how does cascading airflow work exactly?

HVAC Design for USP 797 & 800 Cleanrooms
Not only are healthcare renovations inherently complicated. A pharmacy renovation for USP <797>/<800> compliance adds to the complexity with additional technical challenges associated with higher levels of air filtration, higher air change rates, differential pressure, and additional exhaust requirements.

Where Does USP Chapter 800 Stand Following USP Appeals Panel Decision?
The status of Chapters 795 and 797 is significant in terms of their effect on the applicability of USP General Chapter <800>, which governs the safe handling of hazardous drugs.

Is your Cleanroom Cleanable?
Having cleaning policies and a set routine will help in the long-term sustainability of the cleanroom, but is the room itself and the equipment/furnishings aiding in your efforts, or are they working against you?

Understanding the types of Stainless-Steel in the Pharmacy Cleanroom 304 vs. 316
Not all stainless-steel is the same when it comes to stainless furnishings in the pharmaceutical cleanroom. Knowing and understanding the types of stainless-steel can make the difference on long-term rust and corrosion.

Pharmacy Cleanroom Cleaning Guide
Cleaning and sanitizing pharmacy cleanroom suites and their respective equipment/enclosures to meet USP Chapter 797 and 800 standards requires a high level of attention to detail and the use of well organized and easy to follow protocols.

Implementing “cGMP-Like” Concepts in your 503A Pharmacy Practice
Why would incorporating “cGMP-like” standards and concepts be relevant in your 503A facility?

FDA Revises Guidance on Hospital and Health System Compounding
October 2021 - The Food & Drug Administration (FDA) published a statement that revised its proposed guidance on compounded drugs to help preserve patient access to those medications whose medical needs cannot be met by an FDA-approved drug.

Proposed Revisions to USP General Chapter <797>: Introduction of Category 3 CSP
Earlier this month, the USP announced an extension for public comment for the revisions to USP General Chapters <797> and <795> lasting until January 31st of 2022. Below is a review of the introduction of Category 3 CSPs and how it could impact your pharmacy operations.

Extended Public Comment Period of Proposed Revisions to USP Compounding General Chapters
Wednesday, September 1, 2021: The United States Pharmacopeia (USP) announced the following Compounding General Chapters are available to an extended public comment period until January 31, 2022
<795> Pharmaceutical Compounding – Nonsterile Preparations
<797> Pharmaceutical Compounding – Sterile Preparations
USP Stated: “Revisions to chapters <795> and <797> reflect public health considerations, scientifically robust approaches, and numerous stakeholder engagement activities. The Compounding Expert Committee (CMP EC) engaged healthcare practitioners, regulators, academicians, and other key stakeholders in various sessions including semi-structured interviews with stakeholders, a small roundtable discussion with invited participants, and a broader open forum discussion to collect feedback from a broad range of stakeholders. These engagements helped the CMP EC consider a wide range of perspectives to inform the revisions while maintaining scientific rigor and accounting for today’s public health and practice needs.”

More Than a Box: Effective Training of Cleanroom Personnel for Long-Term Sustainability
No matter how pharmacy cleanrooms are configured and developed, they all serve the same objective, which is to provide an aseptic environment that will protect and secure compounded sterile preparations as well as the pharmacy staff operating within those spaces.
Nevertheless, even the best designed pharmacy cleanrooms will not be successful unless the compounding work force is accurately trained to conduct appropriate behaviors and perform proper cleaning and disinfecting protocols.

More Than a Box: Considerations for Materials and Components on your Pharmacy Cleanroom Build-Out
The difference between a pharmacy cleanroom that must continually undergo repairs and fixes and one that last for years ultimately relies on the type of building materials and components used to create the sterile environment. Design, engineering, and layout all play a heavy factor into the overall functionality and compliance of the space, but daily operations, movement and cleaning are where the materials and components will be tested on their durability.
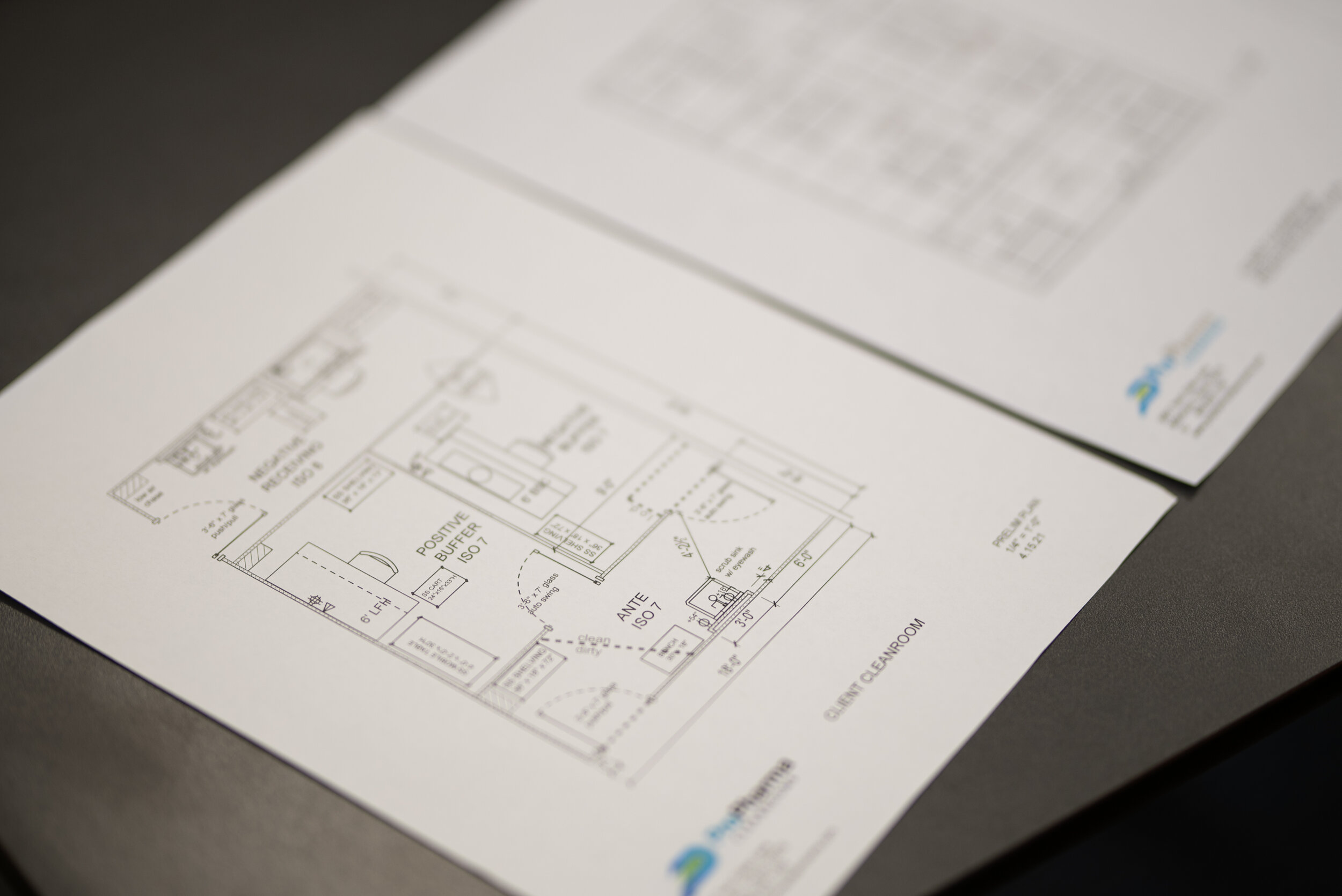
More Than a Box: Pharmacy Cleanroom Design
Pharmacy cleanrooms (for the most part) are almost always confined to small footprint within the overall pharmacy department or work area which makes the design a crucial process to ensure USP and State regulation compliance is met as well as supporting staff and product workflow.
Strict design and build-out standards are laid out in USP General Chapters <797> and <800> that detail proper engineering controls, layout for pressure differentials, as well as equipment and components. All of which can be overwhelming for contractors/architects who might not be familiar with such standards or pharmacy cleanroom projects.
